Fuel Enrichment
5 min read
The energy required by the uranium enrichment method using laser excitation is much lower than other enrichment methods.
The majority of reactors require fuel containing enriched uranium, with a concentration of the fissionable 235U isotope of 3—5% while in natural uranium, it is just 0.7%. Enrichment is a physical process, not a chemical one. It is very demanding regarding both energy and time because atoms differing by a mass of just three neutrons must be separated. The capacity of an enrichment facility is expressed in Separative Work Units (SWU). This is a measure of how much material was processed, the final percentage enrichment, and how much uranium remained in the waste. To produce a single fuel loading requires 140,000 SWU.
Enrichment facilities using gaseous diffusion are in France, the USA, and Russia. Centrifuge enrichment is used in Great Britain, The Netherlands, Germany, Russia, Japan, and other countries.
13,300 tons of silver from the national reserves was lent to the Manhattan project for the winding of the calutron electromagnets.
Calutron
The original enrichment method was developed by Ernest O. Lawrence (1901—1958). It is based on the principle of the mass spectrometer. Inside a calutron, the ions of a tested material move through a magnetic field that deflects their trajectories to form circles. The diameter of the circles depends on the ion’s net charge and mass. In the case of uranium, the trajectories of both isotopes differ minutely and thus the ions strike at different locations. All that is needed is to install a suitable collection tool at the location corresponding to the 235U trajectory and the separation can be accomplished.
Gaseous Diffusion
Gaseous hex is pushed through a semi-permeable membrane. The 235U isotope is somewhat lighter and thus a little bit more will pass through the membrane. About 1,400 cycles are needed to reach the required concentration (3—4%). The majority of the facilities operating on this principle are being phased out. It is expected that they will be decommissioned within the next 20 years. They will be replaced by centrifuges.
Use of the gaseous diffusion principle for uranium enrichment.
All the existing uranium enrichment technologies were developed in the 1940s during the Manhattan Project.
Centrifuges
Video: Model of a cylindrical centrifuge used for uranium enrichment.
After 1960, centrifuges experienced a great boom. A centrifuge looks like a narrow cylinder, 3—5 meters high and only 20 cm in diameter. Gaseous uranium hexafluoride is injected into a centrifuge and when the gas starts to spin at the speed of 50,000 to 70,000 revolutions per minute, the heavier 238U uranium is ejected by the centrifugal force near the walls, while the lighter 235U stays closer to the cylinder’s centerline.
Since the centrifuge bottom heats up, the gas moves upwards and its enriched component is expelled and reused in the subsequent cycle. In this way, the centrifuges can operate continuously. The entire process requires about 10 to 20 cycles to reach the desired enrichment level.
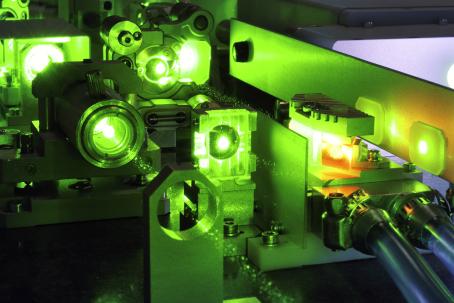
The principle of laser separation of uranium isotopes is based on the ionization of 235U by a precisely tuned laser.
Laser Excitation
In 1970, the development of laser separation of isotopes began in France. Various methods were researched and gradually abandoned until only the Australian Silex remained. Silex is an acronym for Separation of Isotopes by Laser Excitation. A precisely tuned laser that can ionize only the 235U isotope is used to bombard uranium vapor. Positively charged uranium ions are then collected on a negatively charged electrode. This method requires much less energy and thus is also cheaper than the other current enrichment technologies. It is estimated that this method could be up to twenty times more efficient than centrifuge enrichment.
Separation of isotopes by laser excitation may be also used for carbon or silicon enrichment. Certain kinds of these isotopes are required for the development of semiconductor technologies or to produce the oxygen 18O isotope needed for positron emission tomography.